Effect of ligand sequence-specific modification on DNA hybrid catalysis†
Abstract
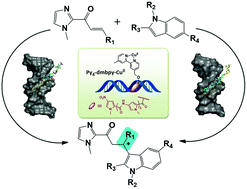
Development of a stereoselective asymmetric catalytic system with high conversion is the key to success for acquiring chiral materials. In recent years, DNA hybrid catalysts have attracted significant interest due to their excellent abilities in accelerating reactions and achieving high enantioselectivity. We report here that bipyridine linked with polyamide as a sequence-specific catalytic ligand was designed to perform a DNA hybrid asymmetric reaction. The products presented different stereoselectivities compared to the results of reactions catalyzed by bipyridine. Comparing catalytic experiments based on alternative oligonucleotides verified that sequence-locating of the ligand affected the catalytic microenvironment. Circular dichroism spectra, combined with singular value decomposition, proposed that different binding modes could exist somewhere between the ligand and alternative sequences. The current work provides a strategy to extend the chemical range of DNA-based catalysis.


 Please wait while we load your content...
Please wait while we load your content...