Synthesis of functionalised azepanes and piperidines from bicyclic halogenated aminocyclopropane derivatives†
Abstract
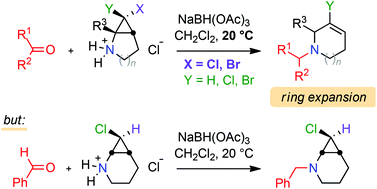
A series of 6,6-dihalo-2-azabicyclo[3.1.0]hexane and 7,7-dihalo-2-azabicyclo[4.1.0]heptane compounds were prepared by the reaction of dihalocarbene species with N-Boc-2,3-dihydro-1H-pyrroles or -1,2,3,4-tetrahydropyridines. Monochloro substrates were synthesised as well, using a chlorine-to-lithium exchange reaction. The behaviour of several aldehydes and ketones under reductive amination conditions with deprotected halogenated secondary cyclopropylamines was investigated, showing that this transformation typically triggers cyclopropane ring cleavage to give access to interesting nitrogen-containing ring-expanded products.


 Please wait while we load your content...
Please wait while we load your content...