Fast & easy preparation of 3D scaffolds from methyl benzoate by a diversity oriented synthesis strategy based on Diels–Alder and ene-reactions†
Abstract
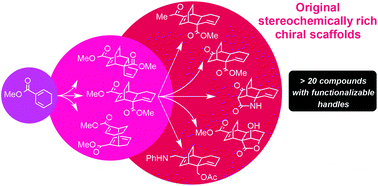
Thermic dimerization of methyl 1,3-cyclohexadiene 2-carboxylate gave original 3D-shape compounds by Diels–Alder cycloaddition and original [6 + 4]-ene reaction. Further selective modifications on an endo [4 + 2] cycloadduct via a diversity oriented synthesis (DOS) strategy quickly led to the preparation of a small library of original 3D scaffolds, providing access to a larger and unexplored chemical space for drug discovery processes.


 Please wait while we load your content...
Please wait while we load your content...