Copper-promoted direct C–H alkoxylation of S,S-functionalized internal olefins with alcohols†
Abstract
Copper-promoted direct C–H alkoxylation of S,S-functionalized internal olefins, that is, α-oxo ketene dithioacetals, was efficiently achieved with alcohols as the alkoxylating agents, (diacetoxyiodo)benzene (PhI(OAc)2) as the oxidant, and benzoquinone (BQ) as the co-oxidant. The alkoxylated olefins were thus constructed and applied for the synthesis of alkoxylated N-heterocycles. The polarization of the olefinic carbon–carbon double bond by the electron-donating dialkylthio and electron-withdrawing α-oxo functionalities plays a crucial role in making such C–H alkoxylation reactions to occur under mild conditions. Mechanistic studies implicate a single-electron-transfer (SET) reaction pathway involved in the overall catalytic cycle.
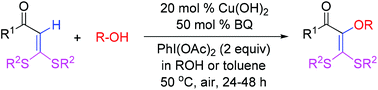


 Please wait while we load your content...
Please wait while we load your content...