Intermolecular sulfenoamination of alkenes with sulfonamides and N-sulfanylsuccinimides to access β-sulfonylamino sulfides and dihydrobenzothiazines†
Abstract
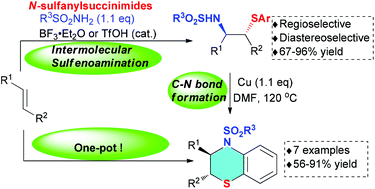
An acid-catalyzed intermolecular sulfenoamination reaction of alkenes is developed with sulfonamides as the nitrogen source and N-sulfanylsuccinimides as the sulfur source. This methodology provides a straightforward and general way to synthesize various β-sulfonylamino sulfides with high regio- and diastereoselectivity. The developed method was coupled with intramolecular C–N coupling in a one-pot procedure to afford a series of dihydrobenzothiazine derivatives, a kind of important heterocycle used as biologically active compounds in medicinal chemistry.


 Please wait while we load your content...
Please wait while we load your content...