Cu(ii)-Mediated keto C(sp3)–H bond α-acyloxylation of N,N-dialkylamides with aromatic carboxylic acids†
Abstract
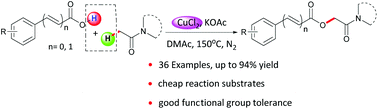
The selective oxidative coupling of aromatic carboxylic acids with the C(sp3)–H bond adjacent to the keto group of alkylamides has been developed by employing a low cost copper source. This provides an efficient approach for synthesis of O-benzoylglycolamides. The protocol displayed good functional group tolerance. A broad range of benzoic acids directly coupled with alkylamides to afford a variety of O-benzoylglycolamides in moderate to good yields. In addition, a reasonable radical mechanism was proposed based on EPR experiments.


 Please wait while we load your content...
Please wait while we load your content...