Enantioselective synthesis of cyclic quaternary α-amino acid derivatives by chiral phosphoric acid catalysis†
Abstract
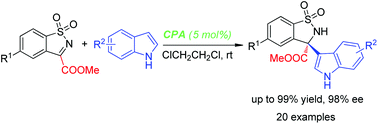
A highly enantioselective aza-Friedel–Crafts reaction of N-sulfonyl cyclic ketimines with indoles catalyzed by chiral phosphoric acids has been developed. This methodology provides an efficient and facile route to indole-containing chiral cyclic α-amino acid derivatives bearing a quaternary stereocenter in high yields and up to 98% enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...