Visible-light mediated directed perfluoroalkylation of hydrazones†
Abstract
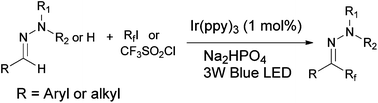
Perfluoroalkylation of N-alkylhydrazones has been achieved via visible light mediated photoredox reactions between the hydrazone and perfluoroalkyl iodide (RfI). This protocol provides a convenient and efficient access to a series of perfluoroalkylated aromatic aldehyde hydrazones which tolerates a wide range of functional groups on the aromatic ring, and allows the use different types of primary and secondary perfluoroalkyl iodides with up to eight carbon atoms. Furthermore, aliphatic aldehyde hydrazones and N-monosubstituted hydrazones which are unreactive in previously reported hydrazone perfluoroalkylation reactions now take part in the reaction under our reaction conditions to give a satisfactory yield of products. Stern–Volmer quenching studies and spin-trapping experiments indicated that these reactions proceed by free radical addition of the Rf radical to the azomethine atom followed by one electron oxidation of the hydrazyl radical and deprotonation of the diazenium cation.


 Please wait while we load your content...
Please wait while we load your content...