Linear and orthogonal peptide templating of silicified protein fibres†
Abstract
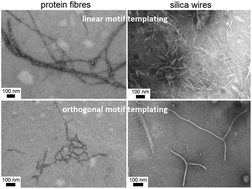
Biomineralisation is essential for biology. Specialist proteins use peptide motifs that catalyse mineral deposition into nano-to-microscale inorganic materials. Unlike in native proteins, the motifs incorporated into self-assembled fibres can persistently propagate on the microscopic scale enabling empirically defined silica nanostructures. Herein we show that the two main modes of motif templating – linear and orthogonal – in self-assembling, fibre-forming peptide sequences effectively silicify protein fibres. We show that the mere charge and morphology of protein fibres are not sufficient for silica deposition, but it is the synergy between fibrillogenesis and silica-specific motifs regularly spaced in fibres that ensures silica templating, regardless of the relative orientation of the motifs.
- This article is part of the themed collection: Peptide Materials


 Please wait while we load your content...
Please wait while we load your content...