Palladium-catalyzed allylation of tautomerizable heterocycles with alkynes†
Abstract
A method for the allylic amidation of tautomerizable heterocycles was developed by a palladium catalyzed allylation reaction with 100% atom economy. A series of structurally diverse N-allylic substituted heterocycles can be synthesized in good yields with high chemo-, regio-, and stereoselectivities under mild conditions.
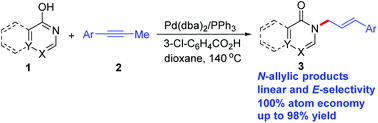


 Please wait while we load your content...
Please wait while we load your content...