Synthesis and biological evaluation of N-arylated-lactam-type iminosugars as potential immunosuppressive agents†
Abstract
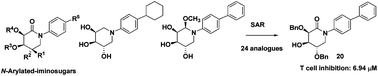
Since the immunosuppressive agents currently used in clinics have significant side effects, it is very important to search for new effective and safe immunosuppressants. Iminosugars as a new class of immunosuppressants are less explored. In this report, 24 new N-arylated iminosugar derivatives, including D-talo and D-galacto epimers, were designed and synthesized, and their immunosuppressive effects were evaluated by MTT assay. The experimental data demonstrated that compound 20 showed the strongest inhibition effect (IC50 = 6.94 μM). Further studies revealed that the inhibitory effects on splenocyte proliferation may come from the suppression of both IFN-γ and IL-4 cytokines. The preliminary structure–activity relationship (SAR) analysis suggested that N-arylated D-galacto-type iminosugars showed better inhibitory activities than D-talo-type analogues. The SAR analysis also showed that the inhibition effect of iminosugars can be improved by decreasing the polarity or increasing the hydrophobicity. These results may be beneficial to the discovery of new iminosugar derivatives as immunosuppressive agents.


 Please wait while we load your content...
Please wait while we load your content...