Base-catalyzed diastereoselective trimerization of trifluoroacetone†
Abstract
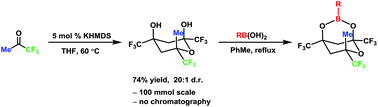
Amphiphilic fluorocarbons have unique properties that facilitate their self assembly and adhesion to both inorganic and biological substrates. Incorporation of these moieties into valuable constructs typically require complex synthetic routes that have limited their use. Here, the base-catalyzed diastereoselective synthesis of 6-methyl-2,4,6-tris(trifluoromethyl)tetrahydro-2H-pyran-2,4-diol is reported. Trimerization of trifluoroacetone in the presence of 5 mol% KHMDS delivers one of four diastereomers selectively in 81% yield with no column chromatography. Temperature screening revealed the reversibility of this trimerization and the funneling of material into the most thermodynamically stable oxane. Subsequent functionalization with boronic acids is reported.


 Please wait while we load your content...
Please wait while we load your content...