Synthesis of seven-membered heterocycles via copper-catalyzed cross-coupling of terminal alkynes with diazo compounds and sequential Michael addition†
Abstract
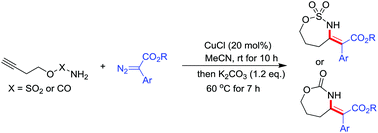
A novel approach to synthesize seven-membered heterocycles is established by reacting amide tethered terminal alkynes with aryl diazoacetates in a one-pot reaction. This reaction involves copper-catalyzed cross coupling followed by base-promoted intramolecular Michael addition and yields products with a medium-sized ring.


 Please wait while we load your content...
Please wait while we load your content...