Chemoenzymatic synthesis of optically active phenolic 3,4-dihydropyridin-2-ones: a way to access enantioenriched 1,4-dihydropyridine and benzodiazepine derivatives†‡
Abstract
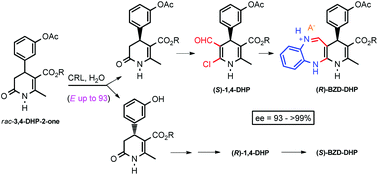
A chemoenzymatic approach for the synthesis of optically active 4-(3-acetoxyphenyl)-5-(alkoxycarbonyl)-6-methyl-3,4-dihydropyridin-2-ones (3,4-DHP-2-ones) and their hydroxyphenyl derivatives has been developed, the key step being a Candida rugosa lipase (CRL)-catalyzed hydrolysis reaction. As a result, different optically active 3,4-DHP-2-ones have been prepared with very high enantiomeric excesses (ee = 94–99%) and good yields. The enantioenriched 3,4-DHP-2-ones have easily been converted into highly functionalized (R)- and (S)-1,4-dihydropyridines (1,4-DHPs) by means of a Vilsmeier–Haack reaction. Finally, the coupling of the 1,4-DHPs with benzene-1,2-diamine using TFA as an acid promoter provided us the corresponding optically active hybrid 1,5-benzodiazepine-1,4-dihydropyridine (BZD-DHP) derivatives. No racemization took place in these processes and all optically active compounds were obtained in excellent yields.


 Please wait while we load your content...
Please wait while we load your content...