Palladium-mediated 11C-carbonylations using aryl halides and cyanamide†
Abstract
A robust and high-yielding radiochemical synthesis of 11C-N-cyanobenzamides using a palladium-mediated aminocarbonylation with 11C-CO, aryl halides and cyanamide is described. The bidentate ligand 1,1′-bis(diphenylphosphino)ferrocene provided 11C-N-cyanobenzamides from aryl-iodides, bromides, triflates and even chlorides in 28–79% radiochemical yield after semi-preparative HPLC. To further highlight the utility of this method, novel 11C-N-cyanobenzamide analogs of flufenamic acid, meflanamic acid, dazoxiben and tamibarotene were synthesized in 34–71% radiochemical yields.
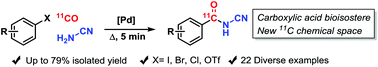


 Please wait while we load your content...
Please wait while we load your content...