A novel metal-free amidosulfenylation of alkenes leading to β-azolyl sulfides†
Abstract
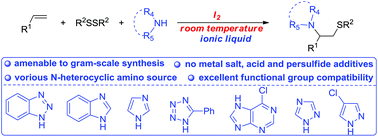
A novel and environmentally benign method for C–S and C–N bond formation by the direct amidosulfenylation of alkenes has been developed under metal-free conditions. Various alkenes and azoles were transformed into the corresponding β-azolyl sulfides in ionic liquids. The wide substrate scope, good functional group tolerance, and ease of operation make this reaction attractive for the synthesis of nitrogen- and sulfur-containing molecules.


 Please wait while we load your content...
Please wait while we load your content...