Display of functional proteins on supramolecular peptide nanofibrils using a split-protein strategy†
Abstract
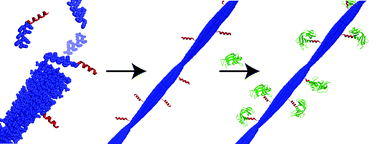
The display of functional proteins on self-assembled peptide nanofibrils is challenging since the steric bulk of proteins attached to simple self-assembling peptides often impedes incorporation into nanofibrils. Herein is described a split-protein strategy to tether functional proteins to preassembled peptide nanofibrils. In this strategy, a short affinity motif peptide derived from a split protein system is appended to a self-assembly motif (the amphipathic Ac-(FKFE)2-NH2 peptide) to form an affinity-assembly fusion peptide. The small size of the affinity motif allows the affinity-assembly fusion peptide to be readily incorporated into peptide nanofibrils that display the affinity motif when the affinity-assembly peptide is coassembled with Ac-(FKFE)2-NH2. Introduction of the split-protein that is complementary to the affinity motif to the assembled nanofibrils results in efficient, multivalent attachment of functional proteins to the peptide nanofibrils. This strategy is demonstrated with two split-protein systems, ribonuclease S′ (RNase S′) and split green fluorescent protein (GFP).
- This article is part of the themed collection: Peptide Materials


 Please wait while we load your content...
Please wait while we load your content...