Stereoselective synthesis of enamino ketones through an aza-Michael/hydrolysis cascade reaction†
Abstract
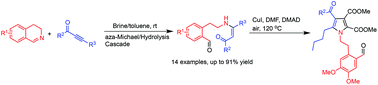
We have developed a mild, convenient and efficient synthesis of highly functionalized (Z)-β-enamino ketones from readily available 3,4-dihydroisoquinoline imines and ynones through an aza-Michael/hydrolysis cascade reaction. This method is also suitable for the preparation of (Z)-β-enamino esters using alkynoates as starting materials. Complex fully substituted pyrroles can be constructed from the obtained (Z)-β-enamino ketones. It is an attractive alternative approach for the preparation of highly functionalized enamino ketones, esters and pyrroles.


 Please wait while we load your content...
Please wait while we load your content...