AlCl3 catalyzed coupling of N-benzylic sulfonamides with 2-substituted cyanoacetates through carbon–nitrogen bond cleavage†
Abstract
A new cross-coupling reaction of N-benzylic sulfonamides with 2-substituted cyanoacetates for the synthesis of 2-substituted benzylbenzene was reported. In the presence of AlCl3, a broad range of N-benzylic sulfonamides reacted smoothly with 2-substituted cyanoacetates to afford structurally diverse benzylbenzenes in moderate to excellent yields. The conversion could be enlarged to gram-scale efficiently. The practicability of this approach was further manifested in the synthesis of a related bioactive agent with high anti-inflammatory activity.
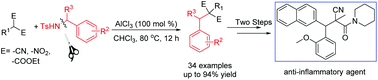


 Please wait while we load your content...
Please wait while we load your content...