Reaction of S-geranyl-2-thiouracil modified oligonucleotides with alkyl amines leads to the N2-alkyl isocytosine derivatives†
Abstract
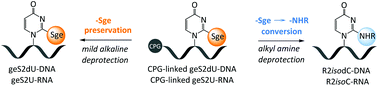
S-Geranylated 2-thiouridines (geS2Us) are unique hydrophobic modified nucleosides identified very recently in bacterial tRNAs. Our study on the synthesis of geS2Ura-containing oligonucleotides (geS2U-RNA and geS2dU-DNA) revealed a fast substitution of the S-geranyl moiety by methylamine (frequently used in oligonucleotide deprotection procedures) or n-butylamine, providing the corresponding N2-alkyl isocytosine (R2isoCyt) derivatives. To retain the S-geranyl moiety in the DNA or RNA chains, the optimized deprotection protocol with 8 M ethanolic ammonia should be applied. The oligomers bearing the R2isoCyt heterocycle (R2isoC-RNA and R2isodC-DNA) are less hydrophobic than the corresponding S2U- and geS2U-modified oligomers, whereas, contrary to the previously reported data, geS2dU-DNA and geS2U-RNA exhibit significantly higher lipophilicity than the parent S2Ura-containing oligonucleotides. Thermodynamic studies revealed that: (a) both geS2Ura- and R2isoCyt-modified oligomers exhibit similar hybridization properties towards DNA and RNA templates, and (b) the R2isoCyt nucleobase preferentially hybridizes to guanine moiety in the DNA/DNA and RNA/RNA duplexes.


 Please wait while we load your content...
Please wait while we load your content...