Aromatic C–H amination: a radical approach for adding new functions into biology- and materials-oriented aromatics
Abstract
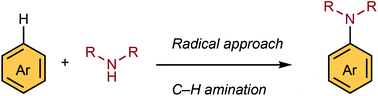
C–H amination is the most powerful method to directly add nitrogen functionalities into a variety of arenes including biology- and materials-oriented molecules. Recent developments in aromatic C–H amination chemistry have enabled the conversion of unactivated arenes into a range of arylamine derivatives without using directing groups or excess amounts of arenes. The key for such successful transformations is the catalytic generation of nitrogen or arene radical intermediates. In this perspective, we discuss recent developments in the radical C–H amination of aromatic molecules. We believe the resulting arylamines, which are hitherto difficult to access, will exhibit unexplored functions for biological and materials application.


 Please wait while we load your content...
Please wait while we load your content...