Application of primary halogenated hydrocarbons for the synthesis of 3-aryl and 3-alkyl indolizines†
Abstract
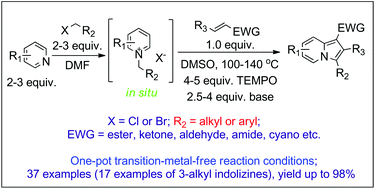
Indolizine is an important heterocyclic compound with several interesting properties that make it suitable for numerous applications in many fields, such as biology, medicine and materials. However, the synthesis of 3-alkyl indolizines from bulky primary halogenated alkanes has not yet been reported. Herein, a transition-metal-free synthetic route to 3-aryl and 3-alkyl indolizines from electron-deficient alkenes, pyridines and primary halogenated hydrocarbons has been reported for the first time using a tandem reaction. The key step of this method is the oxidative dehydrogenative aromatization of a tetrahydroindolizine intermediate with 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO) as the oxidant. The advantages of this protocol are its use of easily available and low-cost starting materials, the transition-metal-free conditions and its ready scalability.


 Please wait while we load your content...
Please wait while we load your content...