Urotensin-II peptidomimetic incorporating a non-reducible 1,5-triazole disulfide bond reveals a pseudo-irreversible covalent binding mechanism to the urotensin G-protein coupled receptor†
Abstract
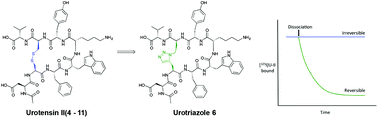
The urotensin-II receptor (UTR) is a class A GPCR that predominantly binds to the pleiotropic cyclic peptide urotensin-II (U-II). U-II is constrained by a disulfide bridge that induces a β-turn structure and binds pseudo-irreversibly to UTR and is believed to result in a structural rearrangement of the receptor. However, it is not well understood how U-II binds pseudo-irreversibly and the nature of the reorganization of the receptor that results in G-protein activation. Here we describe a series of U-II peptidomimetics incorporating a non-reducible disulfide bond structural surrogate to investigate the feasibility that native U-II binds to the G protein-coupled receptor through disulfide bond shuffling as a mechanism of covalent interaction. Disubstituted 1,2,3-triazoles were designed with the aid of computational modeling as a non-reducible mimic of the disulfide bridge (Cys5–Cys10) in U-II. Solid phase synthesis using CuAAC or RuAAC as the key macrocyclisation step provided four analogues of U-II(4–11) incorporating either a 1,5-triazole bridge (5, 6) or 1,4-triazole bridge (9, 10). Biological evaluation of compounds 5, 6, 9 and 10 was achieved using in vitro [125I]UII binding and [Ca2+]i assays at recombinant human UTR. Compounds 5 and 6 demonstrated high affinity (KD ∼ 10 nM) for the UTR and were also shown to bind reversibly as predicted and activate the UTR to increase [Ca2+]i. Importantly, our results provide new insight into the mechanism of covalent binding of U-II with the UTR.


 Please wait while we load your content...
Please wait while we load your content...