Metal-free regioselective formation of C–N and C–O bonds with the utilization of diaryliodonium salts in water: facile synthesis of N-arylquinolones and aryloxyquinolines†
Abstract
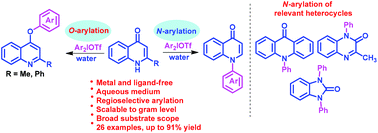
Regioselective construction of crucial C–N and C–O bonds leading to N-arylquinolones and aryloxyquinolines has been accomplished by employing easily accessible diaryliodonium salts and quinolones in water under metal- and ligand-free conditions. This operationally simple strategy is significant due to mild reaction conditions, high product yields, recyclability of released iodoarenes and scalability to the gram level. The practical utility of the developed protocol was proved by the arylation of medicinally important heterocycles like acridin-9(10H)-one, 3-methylquinoxalin-2(1H)-one and 1H-benzo[d]imidazol-2(3H)-one.


 Please wait while we load your content...
Please wait while we load your content...