Copper(i) catalyzed oxidative hydrolysis of Ugi 3-component and Ugi-azide reaction products towards 2° α-ketoamides and α-ketotetrazoles†
Abstract
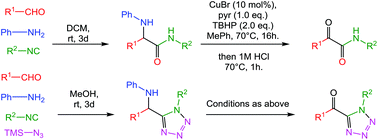
Herein, a two-step MCR-oxidation methodology accessing decorated 2° α-ketoamides and α-ketotetrazoles is described via a catalytic copper(I)-mediated C–N oxidation/acidic hydrolysis of Ugi-three-component and Ugi-azide reaction products. The ability to install diversity from aldehyde and isocyanide synthons allows rapid complexity generation. Of note, (1) 2° α-ketoamides are traditionally difficult to access and more so reminiscent of the endogenous peptide bonds. (2) The route to α-keto-tetrazoles is significantly shorter than that in previous reports.


 Please wait while we load your content...
Please wait while we load your content...