Biosynthetically-inspired oxidations of capillobenzopyranol†
Abstract
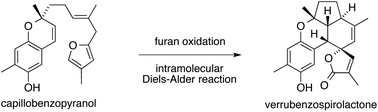
The synthesis of verrubenzospirolactone from its proposed biosynthetic precursor, capillobenzopyranol, has been shown to be chemically feasible via a simple reaction sequence. The key steps are a chemoselective furan oxidation mediated by NaClO2, a stereoselective dehydration of a γ-methoxybutenolide, and an intramolecular Diels–Alder reaction.


 Please wait while we load your content...
Please wait while we load your content...