Camphyl-based α-diimine palladium complexes: highly efficient precatalysts for direct arylation of thiazoles in open-air†
Abstract
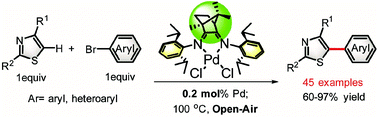
Based on the strategy of the development of phosphine-free palladium-catalyzed direct C–H arylation, a series of camphyl-based α-diimine palladium complexes bearing sterically bulky substituents were synthesized and characterized. The palladium complexes were applied for the cross-coupling of thiazole derivatives with aryl bromides. The effect of the sterically bulky substituent on the N-aryl moiety as well as the reaction conditions was screened. Under the optimal protocols, a wide range of aryl bromides can be smoothly coupled with thiazoles in good to excellent yields in the presence of a low palladium loading of 0.2 mol% under open-air conditions.


 Please wait while we load your content...
Please wait while we load your content...