A flexible 1,2-cis α-glycosylation strategy based on in situ adduct transformation†
Abstract
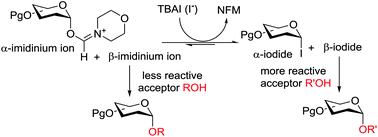
A flexible 1,2-cis α-selective glycosylation strategy for a wide range of glycosyl donors and acceptors has been developed, which is based on an in situ adduct transformation protocol. Based on this strategy, both NFM-derived and iodide covalent adducts can be accessed for glycosylation. Using low temperature NMR spectroscopy, the aforementioned glycosyl adducts were detected.


 Please wait while we load your content...
Please wait while we load your content...