Selective trihydroxylated azepane inhibitors of NagZ, a glycosidase involved in Pseudomonas aeruginosa resistance to β-lactam antibiotics†
Abstract
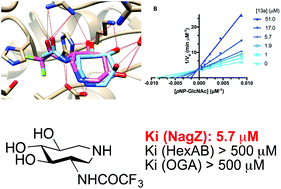
The synthesis of a series of D-gluco-like configured 4,5,6-trihydroxyazepanes bearing a triazole, a sulfonamide or a fluorinated acetamide moiety at C-3 is described. These synthetic derivatives have been tested for their ability to selectively inhibit the muropeptide recycling glucosaminidase NagZ and to thereby increase sensitivity of Pseudomonas aeruginosa to β-lactams, a pathway with substantial therapeutic potential. While introduction of triazole and sulfamide groups failed to lead to glucosaminidase inhibitors, the NHCOCF3 analog proved to be a selective inhibitor of NagZ over other glucosaminidases including human O-GlcNAcase and lysosomal hexosaminidases HexA and B.


 Please wait while we load your content...
Please wait while we load your content...