Divergent synthesis of biflavonoids yields novel inhibitors of the aggregation of amyloid β (1–42)†
Abstract
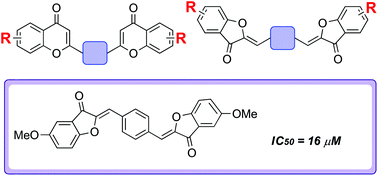
Biflavonoids are associated with a variety of biologically useful properties. However, synthetic biflavonoids are poorly explored within drug discovery. There is considerable structural diversity possible within this compound class and large regions of potentially biologically relevant biflavonoid chemical space remain untapped or underexplored. Herein, we report the development of a modular and divergent strategy towards biflavonoid derivatives which enabled the step-economical preparation of a structurally diverse collection of novel unnatural biflavonoids. Preliminary studies established that the strategy could also be successfully extended to the preparation of very rare triflavonoids, which are also expected to be useful tools for biological intervention. Prompted by previous inhibitory studies with flavonoid libraries, amyloid anti-aggregation screening was performed, which led to the identification of several structurally novel inhibitors of the aggregation of the amyloid β peptide (Aβ42). Aggregated Aβ42 is a pathological hallmark of Alzheimer's disease and the use of small molecules to inhibit the aggregation process has been identified as a potentially valuable therapeutic strategy for disease treatment. Methylated biaurones were associated with highest levels of potency (the most active compound had an IC50 value of 16 μM), establishing this scaffold as a starting point for inhibitor development.


 Please wait while we load your content...
Please wait while we load your content...