Synthesis and structural investigation of 2-aminomethyl-3-(4-methoxy-phenyl)-propionic acid containing a peptide analogue of the amyloidogenic AS(6–7) sequence: inhibition of fibril formation†
Abstract
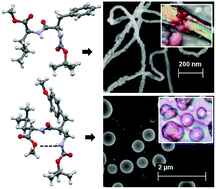
The incorporation of a single β-amino acid moiety in a highly amyloidogenic peptide sequence resulted in the complete inhibition of amyloid fibril formation. The Boc-L-Phe-L-Leu-OMe sequence 1, which has sequence identity with the N-terminal AS(6–7) of the non-immunoglobulin amyloid fibril protein AS, which is responsible for rheumatoid arthritis, self-associates to produce fibrils. The D-Phe analogue peptide 2 shows an elongated ribbon-like morphology. However, the 2-aminomethyl-3-(4-methoxy-phenyl)-propionic acid containing analogue peptide 3 exhibits a polydisperse microsphere morphology. Moreover, fibrils from peptides 1 and 2 exhibit typical green-gold birefringence upon Congo red (CR) staining and show an amyloid-like morphological resemblance. However, the 2-aminomethyl-3-(4-methoxy-phenyl)-propionic acid modified peptide 3 does not respond to the Congo red assay. From X-ray crystallography, peptide 1 with the L-Phe residue adopts an extended structure, whereas the D-Phe analogue 2 adopts a kink-like structure. Both peptides 1 and 2 show twisted anti-parallel sheet-like structures at higher order assembly. However, peptide 3 adopts a nine-membered hydrogen bonded δ-turn-like structure in the solid state and self-associates to form a loop-like supramolecular structure through multiple intermolecular hydrogen bonds. The structural analysis presented herein may foster new studies for de novo design and therapeutics.

 Please wait while we load your content...
Please wait while we load your content...