Organocatalytic decarboxylative aldol reaction of β-ketoacids with α-ketophosphonates en route to the enantioselective synthesis of tertiary α-hydroxyphosphonates†
Abstract
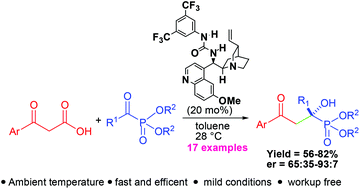
The first example of an asymmetric organocatalyzed decarboxylative aldol reaction of β-ketoacids (aroylacetic acids) with α-ketophosphonates that produces a quaternary chiral centre has been developed. A quinidine based bifunctional urea derivative was identified as the preferred catalyst affording γ-aroyl tertiary α-hydroxyphosphonates in good yield and enantioselectivity. The 31P NMR spectroscopic study was performed to shed light on the reaction mechanism.


 Please wait while we load your content...
Please wait while we load your content...