Alcohol-mediated direct dithioacetalization of alkynes with 2-chloro-1,3-dithiane for the synthesis of Markovnikov dithianes†
Abstract
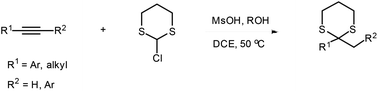
An alcohol-mediated dithioacetalization process that gains direct access to the corresponding Markovnikov-selective 1,3-dithianes using unactivated alkynes and nonthiolic/odorless 2-chloro-1,3-dithiane in a highly efficient manner has been developed. This methodology has the advantage of having mild reaction conditions, and the dithioacetalization process gives good to excellent yields with high Markovnikov-selectivity.


 Please wait while we load your content...
Please wait while we load your content...