“Anti-Michael addition” of Grignard reagents to sulfonylacetylenes: synthesis of alkynes†
Abstract
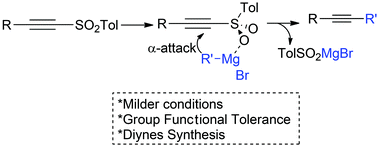
In this work, the addition of Grignard reagents to arylsulfonylacetylenes, which undergoes an “anti-Michael addition”, resulting in their alkynylation under very mild conditions is described. The simplicity of the experimental procedure and the functional group tolerance are the main features of this methodology. This is an important advantage over the use of organolithium at −78 °C that we previously reported. Moreover, the synthesis of diynes and other examples showing functional group tolerance in this anti-Michael reaction is also presented.


 Please wait while we load your content...
Please wait while we load your content...