Fluorous-tag assisted synthesis of bile acid–bisphosphonate conjugates via orthogonal click reactions: an access to potential anti-resorption bone drugs†
Abstract
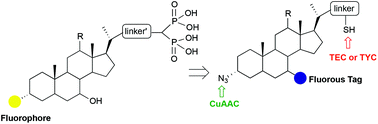
The synthesis of a small collection of novel bile acid–bisphosphonate (BA–BP) conjugates as potential drug candidates is reported. The disclosed methodology relied on the installation of azide and thiol functionalities at the head and tail positions, respectively, of the BA scaffold and its subsequent decoration by orthogonal click reactions (copper-catalyzed azide–alkyne cycloaddition, thiol–ene or thiol–yne coupling) to introduce BP units and a fluorophore. Because of the troublesome isolation of the target conjugates by standard procedures, the methodology culminated with the functionalization of the BA scaffold with a light fluorous tag to rapidly and efficiently purify intermediates and final products by fluorous solid-phase extraction.


 Please wait while we load your content...
Please wait while we load your content...