Pd(0)-Catalysed asymmetric reductive Heck-type cyclization of (Z)-1-iodo-1,6-dienes and enantioselective synthesis of quaternary tetrahydropyridines†
Abstract
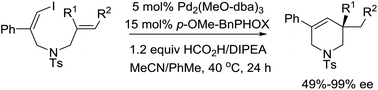
A Pd(0)/(S)-p-MeO-BnPHOX catalytic system has been established for the asymmetric reductive Heck reaction of (Z)-1-iodo-1,6-dienes, which affords quaternary tetrahydropyridines with good to excellent enantioselectivities. This reaction tolerates a wide range of substituted alkene moieties, including 1,1-disubstituted, 1,1,2-trisubstituted as well as 1,2-disubstituted alkenes.


 Please wait while we load your content...
Please wait while we load your content...