Selective construction of quaternary stereocentres in radical cyclisation cascades triggered by electron-transfer reduction of amide-type carbonyls†
Abstract
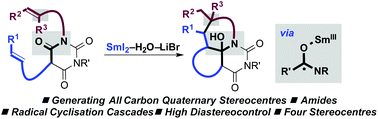
Radical–radical cyclisation cascades, triggered by single-electron-transfer to amide-type carbonyls using SmI2–H2O–LiBr, result in the selective construction of quaternary carbon stereocentres. The cascades deliver tricyclic barbiturates with four stereocentres in good yield and with excellent diastereocontrol.
- This article is part of the themed collection: Polycyclic Reactions in Synthesis and Biosynthesis


 Please wait while we load your content...
Please wait while we load your content...