Zinc-mediated α-regioselective Barbier-type cinnamylation reactions of aldehydes, ketones and esters†
Abstract
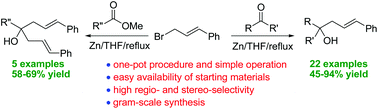
We report a simple, efficient, and general method for the zinc-mediated regioselective cinnamylation of aldehydes and ketones under Barbier-type conditions in a one-pot synthesis affording the corresponding α-cinnamylated alcohols in moderate to excellent yields. Compared to the literature procedures, this approach is operationally simple, uses simple reactants, and provides direct access to linear α-cinnamylated alcohols with excellent regioselectivity. Experimental results suggest that the reactions proceed through the radical pathway. In addition, the reaction was found to be scalable to the gram-scale and the one-pot protocol is also applicable to less reactive esters leading to bishomoallylic alcohols which were valuable intermediates for desymmetrizing intramolecular Heck cyclization, allowing for the elaboration to functionalized building blocks.


 Please wait while we load your content...
Please wait while we load your content...