FeCl3 catalysed 7-membered ring formation in a single pot: a new route to indole-fused oxepines/azepines and their cytotoxic activity†
Abstract
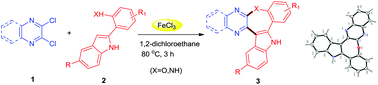
Various oxepine and azepine fused N-heterocyclic derivatives were synthesized using a new and one-pot reaction of 2,3-dichloro quinoxaline/pyrazine with 2-(1H-indol-2-yl)phenol/aniline in the presence of 25 mol% FeCl3. The reaction proceeded via C–C bond followed by C–X (X = O or N) bond formation to construct the central 7-membered ring, affording the desired products in good yields. The structure assignment was confirmed by the single crystal X-ray analysis of a synthesized oxepine fused N-heterocycle derivative. Most of the synthesized compounds were found to be promising when tested for their anti-proliferative properties against cervical and breast cancer cell lines.


 Please wait while we load your content...
Please wait while we load your content...