A new four-component reaction involving the Michael addition and the Gewald reaction, leading to diverse biologically active 2-aminothiophenes†
Abstract
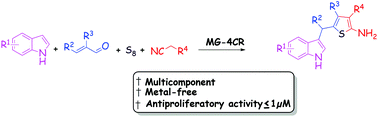
The Gewald three-component reaction yielding highly substituted 2-aminothiophene heterocycles has been known for a long time and holds an extraordinary potential in the pharmaceutical industry. Herein, we describe a four-component reaction initiated by the conjugate addition of different indole derivatives to α,β-unsaturated carbonyl compounds. This is followed by an in situ Gewald three-component reaction which results in the formation of a compound containing an indole and a 2-aminothiophene moiety separated by a methylene spacer. We also examined the impact of the use of other nucleophilic components such as pyrrole derivatives on this MG-4CR (Michael–Gewald four component reaction). All these synthesized compounds were tested for anti-proliferative activity and three of them showed activity in the nanomolar range.


 Please wait while we load your content...
Please wait while we load your content...