1,5-Electrocyclization of conjugated azomethine ylides derived from 3-formyl chromene and N-alkyl amino acids/esters†
Abstract
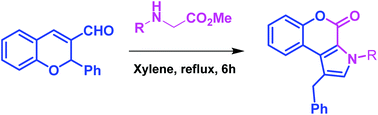
A novel strategy has been developed for the synthesis of chromeno[3,4-b]pyrrol-4(3H)-one and substituted pyrrole derivatives through 1,5-electrocyclization of conjugated azomethine ylides. This is the first example of the preparation of highly substituted pyrrole derivatives from chromene-3-carboxaldehydes (non-enolizable aldehydes) and N-alkyl amino acids/esters. This method is simple and applicable to a diverse range of substrates.


 Please wait while we load your content...
Please wait while we load your content...