Construction of polycyclic frameworks via a DMAP-catalysed tandem addition–(4 + 2) annulation sequence of δ-acetoxy allenoate†
Abstract
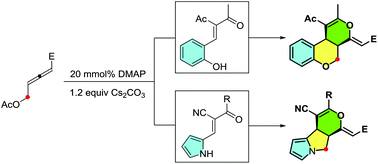
Here is reported the DMAP-catalyzed addition/(4 + 2) annulation domino reaction of δ-acetoxy allenoate with either salicylaldehyde-derived oxadiene or pyrrolealdehyde-derived oxadiene, which provides a facile method toward polycyclic frameworks. A cationic intermediate, 3-ammonium-2,4-dienoate, is proposed to be involved via an addition–elimination process between an allenoate and a catalyst, which is capable of undergoing addition with either O- or N-nucleophile and subsequent (4 + 2) annulation with oxadiene.


 Please wait while we load your content...
Please wait while we load your content...