High-affinity recognition of the human C-reactive protein independent of phosphocholine†
Abstract
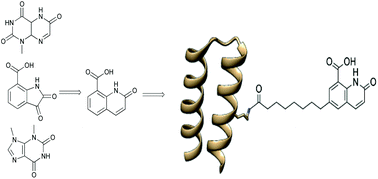
A high-affinity polypeptide conjugate 4-C25L22-DQ, has been developed for the molecular recognition of the human C-reactive protein, CRP, a well-known inflammation biomarker. CRP is one of the most frequently quantified targets in diagnostic applications and a target in drug development. With the exception of antibodies, most molecular constructs take advantage of the known affinity for CRP of phosphocholine that depends on Ca2+ for its ability to bind. 4-C25L22-DQ which is unrelated to phosphocholine binds in the absence of Ca2+ with a dissociation constant of 760 nM, an order of magnitude lower than that of phosphocholine, the KD of which is 5 μM. The small organic molecule 2-oxo-1,2-dihydroquinoline-8-carboxylic acid (DQ) was designed based on the structural similarities between three hits from a set of compounds selected from a building block collection and evaluated with regards to affinity for CRP by NMR spectroscopy. 4-C25L22-DQ was shown in a competition experiment to bind CRP three orders of magnitude more strongly than DQ itself, and in a pull-down experiment 4-C25L22-DQ was shown to extract CRP from human serum. The development of a robust and phosphocholine-independent recognition element provides unprecedented opportunities in bioanalytical applications in vivo and in vitro under conditions where the concentration of Ca2+ ions is low, or where Ca2+ binding agents such as EDTA or heparin are needed to prevent blood coagulation. The identification from a compound library of a small organic molecule and its conjugation to a small set of polypeptides, none of which were previously known to bind CRP, illustrates a convenient and general route to selective high-affinity binders for proteins with dissociation constants in the μM to nM range for which no small molecule ligands are known.


 Please wait while we load your content...
Please wait while we load your content...