Asymmetric conjugate additions of 2-substituted benzofuran-3(2H)-ones to α,β-unsaturated ketones catalyzed by chiral copper complexes†
Abstract
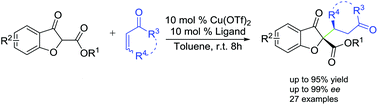
A highly enantioselective conjugate addition of 2-substituted benzofuran-3(2H)-ones to α,β-unsaturated ketones promoted by chiral copper complexes has been developed, affording the Michael addition products with quaternary stereocenters in good to high yields (up to 95% yield) with excellent enantioselectivities (up to 99% ee). The chiral Michael adducts could be readily converted to the polycyclic benzofuran-type framework via the Robinson annulation.


 Please wait while we load your content...
Please wait while we load your content...