Solvent-free, uncatalyzed asymmetric “ene” reactions of N-tert-butylsulfinyl-3,3,3-trifluoroacetaldimines: a general approach to enantiomerically pure α-(trifluoromethyl)tryptamines†
Abstract
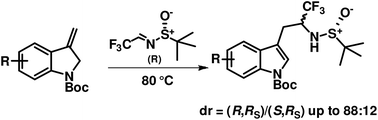
A novel approach to regioselectively substituted and stereoselectively α-trifluoromethylated tryptamines is reported based on the ene reaction of Boc-protected 3-methyleneindolines with optically pure (R)- or (S)-tert-butanesulfinyltrifluoroacetaldimine. Boc- and sulfinylamido-protected α-trifluoromethyltryptamines are obtained in 60–70% yield and 85/15 dr by just heating equimolar amounts of the two reaction partners at 80–90 °C for 2–3 h without a solvent. The absolute configuration of the amino α-carbon has been assigned based on the vibrational circular dichroism (VCD) spectral analysis. The two protecting group can be chemoselectively removed allowing further regio- and stereoselective elaboration of the ene products to various biologically interesting compounds.


 Please wait while we load your content...
Please wait while we load your content...