Signal transduction in oligoamide foldamers by selective non-covalent binding of chiral phosphates at a urea binding site†
Abstract
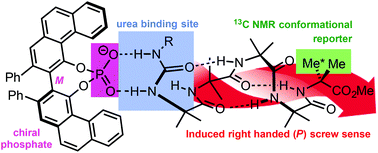
The transduction of biological signals depends on the spatial communication of conformational change. We report a synthetic mimic of this signal transduction process in which non-covalent binding induces a change in the position of equilibrium between two rapidly interconverting screw-sense conformers of a synthetic helical polyamide. Selectivity was achieved by incorporating at the N-terminus of the polyamide a urea-based anion recognition site capable of binding chiral phosphate anions. As a result of solvent-dependent binding, an induced conformational change propagates from the binding site through the amide chain, leading to a screw-sense preference detectable in the form of a chemical shift separation between two NMR active 13C labels. The remote induction of screw sense preference indicates successful communication of a signal originating solely from non-covalent binding.


 Please wait while we load your content...
Please wait while we load your content...