Triamide macrocyclic chloride receptors via a one-pot tandem reduction–condensation–cyclization reaction†
Abstract
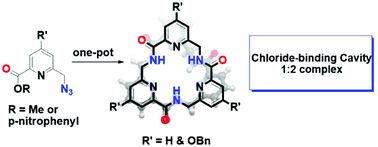
A pyridine containing triamide macrocycle and its substituted analog have been synthesized in one pot from the corresponding monomer without the use of coupling reagents. The macrocycle can selectively bind chloride ions. The ease of synthesis and chloride-binding properties of the macrocycle make it a highly attractive scaffold for ion-encapsulation, ion-transport and water purification.


 Please wait while we load your content...
Please wait while we load your content...