Metal-free synthesis of 2-aminonaphthalenes by intramolecular transannulation of 1-sulfonyl-4-(2-alkenylphenyl)-1,2,3-triazoles†
Abstract
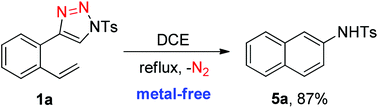
A facile metal-free synthesis of naphthalenes by intramolecular transannulation of 1-sulfonyl-4-(2-alkenylphenyl)-1,2,3-triazoles was realized. The in situ formed ketenimine was proposed as the key intermediate, and the desired 2-aminonaphthalenes were generated in up to 87% yield in refluxing 1,2-dichloroethane without any catalyst or additive.


 Please wait while we load your content...
Please wait while we load your content...