Iodine-mediated synthesis of sulfur-bridged enaminones and chromones via double C(sp2)–H thiolation†
Abstract
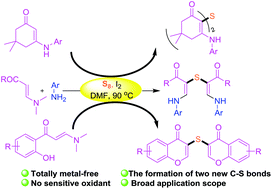
The reactions of various enaminones with elemental sulfur giving rise to both sulfur-bridged enaminones and chromones have been realized via iodine promotion. All products were furnished by means of double C(sp2)–H bond thiolation without using any metal catalyst or sensitive oxidant, providing a simple and efficient protocol for the synthesis of diverse sulfur derivatives of enaminones.


 Please wait while we load your content...
Please wait while we load your content...