Regioselective dehydration of α-hydroxymethyl tetrahydrofurans using Burgess’ reagent under microwave irradiation†
Abstract
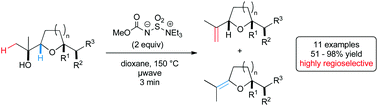
We here present a highly efficient and high yielding procedure for the preparation of 2-vinyl tetrahydrofurans starting from α-hydroxymethyl tetrahydrofurans. Best results for this dehydration were achieved using Burgess’ reagent in dioxane under microwave irradiation. A range of functional groups as well as different cyclic and bicyclic frameworks were found to be compatible with the reaction conditions. The desired products were obtained within minutes in good to high yields and excellent regioselectivity.


 Please wait while we load your content...
Please wait while we load your content...